
Injectafer® (ferric carboxymaltose
injection) improves exercise
capacity for patients with ID and HF1
Choose Injectafer
To provide the most iron per course of treatment in
IDA1-6*† & For Exercise Capacity in iron deficiency (ID) and
heart failure (HF)1‡
- The most-studied intravenous (IV) iron in the world7§
- Over 3 million US patients treated7||

Connect with American Regent for more information
Replenish your patients’ iron deficit with Injectafer (ferric carboxymaltose injection)
When oral iron fails, it may be time to consider an IV iron for your patients with IDA
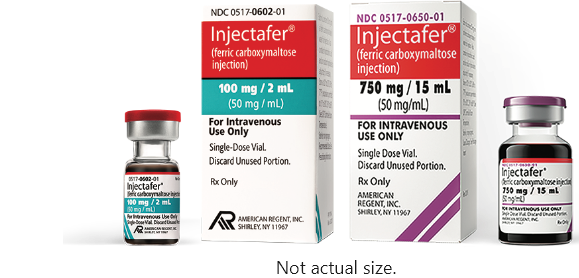
Choose Injectafer (ferric carboxymaltose injection), the only FDA-approved IV iron that restores up to 1500 mg in 1 course of therapy. Injectafer is dosed in 2 administrations of 750 mg separated by at least 7 days.1 100% of IV iron is delivered into your patient's bloodstream. Injectafer is also available as a 100 mg iron/2 mL single-dose vial.1
 Artist's rendering.
Artist's rendering.
1500 mg in one course of treatment#**


IV infusion over at least 15 minutes
Slow IV push over 7.5 minutes
**When administered via IV infusion, dilute up to 750 mg of iron in no more than 250 mL of sterile 0.9% sodium chloride injection, USP, such that the concentration of the infusion is not <2 mg of iron per mL, and administer over at least 15 minutes. When administered as a slow IV push, give at the rate of approximately 100 mg (2 mL) per minute.1
Recommended weight-based dosing for adult patients with ID in HF1
After determining your adult patients with HF and NYHA class II/III have ID, calculate the total iron needed using the dosing tables below1
| DAY 1 | |
| Hb (g/dL) |
Patient body weight <70 kg or ≥70 kg |
| ≤14 | 1000 mg |
| ≥14* | 500 mg |
*There are no data available to guide Injectafer dosing in patients with Hb ≥15.
| WEEK 6† | ||||||
| Hb (g/dL) |
|
|||||
| <10 | 500 mg | 1000 mg | ||||
| ≥10* | No dose | 500 mg | ||||
*There are no data available to guide Injectafer dosing in patients with Hb ≥15.
†No week 6 dose is needed for patients with a Hb (g/dL) ≥14.
| WEEK 12, 24, AND 36‡ |
|
Administer a maintenance dose of 500 mg, if serum ferritin <100 ng/mL or serum ferritin 100 ng/mL to 300 ng/mL with Transferrin Saturation (TSAT) <20% |
‡There are no data available to guide Injectafer dosing past 36 weeks.
For your adult patients with IDA who have either intolerance or an unsatisfactory response to oral iron, continue to use 2 doses of 750 mg separated by at least 7 days. For your pediatric patients 1 year of age and older with IDA use the weight based dosing in the Injectafer Prescribing Information1§II¶
A majority of patients with HF received 1500 mg or more of Injectafer (ferric carboxymaltose injection)1,8
In the Injectafer (ferric carboxymaltose injection) pivotal trials for the treatment of IDA, a fixed-dose regimen of 1500 mg was used.1 In CONFIRM-HF (a trial specific to ID treatment in certain HF patients to determine whether intravenous Injectafer improves exercise capacity), the mean and median total dose was 1500 mg (with a dosing range of 500 mg-3500 mg iron) to determine if intravenous Injectafer improves exercise capacity.8
||When administered via infusion, dilute up to 750 mg of iron in no more than 250 mL of sterile 0.9% sodium chloride injection, USP, such that the concentration of the infusion is not <2 mg of iron per mL, and administer over at least 15 minutes. When administering Injectafer 500 mg or 750 mg as a slow IV push, give at the rate of approximately 100 mg (2 mL) per minute.1,7 ¶Injectafer is a first-line treatment for adult patients with IDA who have Non-Dialysis Dependent Chronic Kidney Disease (NDD-CKD).1
Diagnosing and Treating IDA
A wide variety of patient types should be tested for IDA
IDA and cancer
There is a 44% to 50% prevalence of IDA across tumor types, including hematological malignancies.9
IDA and ID in HF
~50% of all patients with HF have ID.12
17% of patients with chronic HF have ID and anemia13
IDA and NDD-CKD
57.88%–58.8% (of men) and 69.9%–72.8% (of women) with NDD-CKD have low iron tests (NHANES III).14‡‡ Injectafer may be used as first-line treatment in adult patients with NDD-CKD.1
InjectConnect™ Support Program

Contact Daiichi Sankyo
Access Central
Daiichi Sankyo Access Central offers a suite of support programs to help appropriate patients access Injectafer therapy, including claims & appeals support and financial assistance programs.
Visit DSIAccessCentral.com to download helpful resources. You can also connect live with an Access Central Coordinator by calling 1-866-4-DSI-NOW, Monday–Friday, 8:00 AM–6:00 PM ET.
Financial Assistance Programs
Injectafer Savings Program
Financial Assistance§§
Co-pay savings may help reduce commercially insured patients out-of-pocket costs||||
PATIENTS RECEIVE EACH
DOSE FOR AS LITTLE AS
For eligible patients
- Assistance of up to $500 per dose
- Enrollment is valid for 2 courses of treatment per 12-month period
Patient Assistance Program
The Patient Assistance Program was created to help patients who lack health insurance or are commercially underinsured and cannot afford therapy.
For more information, including how to apply for your patients, visit DSIAccessCentral.com.
Injectafer Savings Program Terms and Conditions
- This offer is valid for commercially insured patients. Uninsured and cash-paying patients are NOT eligible for this Program.
- Depending on insurance coverage, eligible patients may pay no more than $50 per dose for up to four doses per calendar year. There is a maximum savings limit of $500 per dose, with an overall program limit of $2,000 per calendar year. Check with your pharmacist or healthcare provider for your co-pay discount. Patient out-of-pocket expense may vary.
- This offer is not valid for patients enrolled in Medicare Part B or Medicare Part D, Medicaid, or other federal or state healthcare programs, or private indemnity or HMO insurance plans that reimburse you for the entire cost of your prescription drugs. Patients may not use this card if they are Medicare-eligible and enrolled in an employer-sponsored health plan or medical or prescription drug benefit program for retirees.
- An explanation of benefits (EOB) statement must be faxed, uploaded in the portal, or mailed in prior to transacting on the account numbers for co-pay assistance.
- Offer is invalid for claims or transactions more than 180 days from the date on the EOB.
- Patients will be automatically re-enrolled in the next calendar year. If there is no copay claim activity for 18 months, the enrollment will be canceled.
- Daiichi Sankyo, Inc. reserves the right to rescind, revoke or amend this offer without notice. Offer good only in the USA, including Puerto Rico, at participating pharmacies or healthcare providers.
- Void if prohibited by law, taxed, or restricted.
- This account number is not transferable. The selling, purchasing, trading, or counterfeiting of this account number is prohibited by law.
- This account number is not insurance.
- By redeeming this account number, you acknowledge that you are an eligible patient and that you understand and agree to comply with the terms and conditions of this offer.
- Qualified patients receiving Injectafer will be allowed a 180-day retroactive enrollment period from the date of EOB (eligibility of benefit form) to receive benefits under the program rules.
Patient Assistance Program – Program Eligibility
Patients must meet all of the following to be eligible for the program: 1) meet established income requirements, 2) lack health insurance completely or be commercially underinsured, and 3) be a resident of the USA or its territories, including Puerto Rico.
References:
- Injectafer®. Package insert. American Regent, Inc; 2025.
- Venofer®. Package insert. American Regent, Inc; 2022.
- Ferrlecit®. Package insert. sanofi-aventis U.S. LLC; 2022.
- INFeD®. Package insert. Allergan USA, Inc; 2021.
- Feraheme®. Package insert. AMAG Pharmaceuticals, Inc; 2022.
- Monoferric®. Package insert. Pharmacosmos Therapeutics Inc; 2022.
- Data on File. American Regent. Shirley, NY.
- Ponikowski P, van Veldhuisen DJ, Comin-Colet J, et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J. 2015;36(11):657-668. doi:10.1093/eurheartj/ehu385
- Ludwig H, Müldür E, Endler G, Hübl W. Prevalence of iron deficiency across different tumors and its association with poor performance status, disease status and anemia. Ann Oncol. 2013;24(7):1886-1892. doi:10.1093/annonc/mdt118
- Your guide to anemia. National Heart, Lung, and Blood Institute. Published September 2011. Accessed August 23, 2023. https://www.nhlbi.nih.gov/sites/default/files/publications/11-7629.pdf
- Stein J, Hartmann F, Dignass AU. Diagnosis and management of iron deficiency anemia in patients with IBD. Nat Rev Gastroenterol Hepatol. 2010;7(11):599-610. doi:10.1038/nrgastro.2010.151
- Ebner N, von Haehling S. Why is iron deficiency recognized as an important comorbidity in heart failure? Card Fail Rev. 2019;5(3):173-175. doi:10.15420/cfr.2019.9.2
- Klip IT, Comin-Colet J, Voors AA, et al. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J. 2013;165(4):575-582.e3. doi:10.1016/j.ahj.2013.01.017
- Fishbane S, Pollack S, Feldman HI, Joffe MM. Iron indices in chronic kidney disease in the National Health and Nutritional Examination Survey 1988–2004. Clin J Am Soc Nephrol. 2009;4(1):57-61.
-
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
Injectafer is contraindicated in patients with hypersensitivity to Injectafer or any of its inactive components.
WARNINGS AND PRECAUTIONS
Symptomatic Hypophosphatemia
Symptomatic hypophosphatemia with serious outcomes including osteomalacia and fractures requiring clinical intervention has been reported in patients treated with Injectafer in the post-marketing setting. These cases have occurred mostly after repeated exposure to Injectafer in patients with no reported history of renal impairment. However, symptomatic hypophosphatemia has been reported after one dose. Possible risk factors for hypophosphatemia include a history of gastrointestinal disorders associated with malabsorption of fat-soluble vitamins or phosphate, inflammatory bowel disease, concurrent or prior use of medications that affect proximal renal tubular function, hyperparathyroidism, vitamin D deficiency, malnutrition, and hereditary hemorrhagic telangiectasia (HHT or Osler-Weber-Rendu syndrome). In most cases, hypophosphatemia resolved within three months.
Correct pre-existing hypophosphatemia prior to initiating therapy with Injectafer. Monitor serum phosphate levels in patients at risk for chronic low serum phosphate. Check serum phosphate levels prior to a repeat course of treatment in patients at risk for low serum phosphate and in any patient who receives a second course of therapy within three months. Treat hypophosphatemia as medically indicated.
Hypersensitivity Reactions
Serious hypersensitivity reactions, including anaphylactic-type reactions, some of which have been life-threatening and fatal, have been reported in patients receiving Injectafer. Patients may present with shock, clinically significant hypotension, loss of consciousness, and/or collapse. Monitor patients for signs and symptoms of hypersensitivity during and after Injectafer administration for at least 30 minutes and until clinically stable following completion of the infusion. Only administer Injectafer when personnel and therapies are immediately available for the treatment of serious hypersensitivity reactions. In clinical trials, serious anaphylactic/anaphylactoid reactions were reported in 0.1% (2/1775) of subjects receiving Injectafer. Other serious or severe adverse reactions potentially associated with hypersensitivity which included, but were not limited to, pruritus, rash, urticaria, wheezing, or hypotension were reported in 1.5% (26/1775) of these subjects.
Hypertension
In clinical studies, hypertension was reported in 4% (67/1775) of subjects in clinical trials 1 and 2. Transient elevations in systolic blood pressure, sometimes occurring with facial flushing, dizziness, or nausea were observed in 6% (106/1775) of subjects in these two clinical trials. These elevations generally occurred immediately after dosing and resolved within 30 minutes. Monitor patients for signs and symptoms of hypertension following each Injectafer administration.
Laboratory Test Alterations
In the 24 hours following administration of Injectafer, laboratory assays may overestimate serum iron and transferrin bound iron by also measuring the iron in Injectafer.
ADVERSE REACTIONS
Adults
In two randomized clinical studies [Studies 1 and 2], a total of 1775 patients were exposed to Injectafer, 15 mg/kg of body weight, up to a maximum single dose of 750 mg of iron on two occasions, separated by at least 7 days, up to a cumulative dose of 1500 mg of iron. Adverse reactions reported by >2% of Injectafer-treated patients were nausea (7.2%); hypertension (4%); flushing (4%); injection site reactions (3%); erythema (3%); hypophosphatemia (2.1%); and dizziness (2.1%).
Pediatric
The safety of Injectafer in pediatric patients was evaluated in Study 3. Study 3 was a randomized, active-controlled study in which 40 patients (1 to 12 years of age: 10 patients, 12 to 17 years of age: 30 patients) received Injectafer 15 mg/kg to a maximum single dose of 750 mg (whichever was smaller) on Days 0 and 7 for a maximum total dose of 1500 mg; 38 patients evaluable for safety in the control arm received an age-dependent formulation of oral ferrous sulfate for 28 days. The median age of patients who received Injectafer was 14.5 years (range, 1-17); 83% were female; 88% White and 13% Black. The most common adverse reactions (≥4%) were hypophosphatemia (13%), injection site reactions (8%), rash (8%), headache (5%), and vomiting (5%).
Patients with Iron Deficiency and Heart Failure
The safety of Injectafer was evaluated in adult patients with iron deficiency and heart failure in randomized controlled trials FAIR-HF (NCT00520780), CONFIRM-HF (NCT01453608) and AFFIRM-AHF (NCT02937454) in which 1016 patients received Injectafer versus 857 received placebo. The overall safety profile of Injectafer was consistent across the studied indications.
Post-Marketing Experience
The following adverse reactions have been identified during post approval use of Injectafer. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following adverse reactions have been reported from the post-marketing spontaneous reports with Injectafer: cardiac disorders: tachycardia; general disorders and administration site conditions: chest discomfort, chills, pyrexia; metabolism and nutrition disorders: hypophosphatemia; musculoskeletal and connective tissue disorders: arthralgia, back pain, hypophosphatemic osteomalacia; nervous system disorders: syncope; respiratory, thoracic and mediastinal disorders: dyspnea; skin and subcutaneous tissue disorders: angioedema, erythema, pruritus, urticaria; pregnancy: fetal bradycardia.
USE IN SPECIFIC POPULATIONS
Pregnancy – Fetal/Neonatal Adverse Reactions
Severe adverse reactions including circulatory failure (severe hypotension, shock including in the context of anaphylactic reaction) may occur in pregnant women with parenteral iron products (such as Injectafer) which may cause fetal bradycardia, especially during the second and third trimester.
INDICATIONS
Injectafer® (ferric carboxymaltose injection) is indicated for the treatment of iron deficiency anemia (IDA) in adult and pediatric patients 1 year of age and older who have either intolerance or an unsatisfactory response to oral iron, and in adult patients who have non-dialysis dependent chronic kidney disease. Injectafer is also indicated for iron deficiency in adult patients with heart failure and New York Heart Association class II/III to improve exercise capacity.
You are encouraged to report Adverse Drug Events to American Regent, Inc. at 1-800-734-9236 or to the FDA by visiting www.fda.gov/medwatch or calling 1-800-FDA-1088.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
Injectafer is contraindicated in patients with hypersensitivity to Injectafer or any of its inactive components.
WARNINGS AND PRECAUTIONS
Symptomatic Hypophosphatemia
Symptomatic hypophosphatemia with serious outcomes including osteomalacia and fractures requiring clinical intervention has been reported in patients treated with Injectafer in the post-marketing setting. These cases have occurred mostly after repeated exposure to Injectafer in patients with no reported history of renal impairment. However, symptomatic hypophosphatemia has been reported after one dose. Possible risk factors for hypophosphatemia include a history of gastrointestinal disorders associated with malabsorption of fat-soluble vitamins or phosphate, inflammatory bowel disease, concurrent or prior use of medications that affect proximal renal tubular function, hyperparathyroidism, vitamin D deficiency, malnutrition, and hereditary hemorrhagic telangiectasia (HHT or Osler-Weber-Rendu syndrome). In most cases, hypophosphatemia resolved within three months.
Correct pre-existing hypophosphatemia prior to initiating therapy with Injectafer. Monitor serum phosphate levels in patients at risk for chronic low serum phosphate. Check serum phosphate levels prior to a repeat course of treatment in patients at risk for low serum phosphate and in any patient who receives a second course of therapy within three months. Treat hypophosphatemia as medically indicated.
Hypersensitivity Reactions
Serious hypersensitivity reactions, including anaphylactic-type reactions, some of which have been life-threatening and fatal, have been reported in patients receiving Injectafer. Patients may present with shock, clinically significant hypotension, loss of consciousness, and/or collapse. Monitor patients for signs and symptoms of hypersensitivity during and after Injectafer administration for at least 30 minutes and until clinically stable following completion of the infusion. Only administer Injectafer when personnel and therapies are immediately available for the treatment of serious hypersensitivity reactions. In clinical trials, serious anaphylactic/anaphylactoid reactions were reported in 0.1% (2/1775) of subjects receiving Injectafer. Other serious or severe adverse reactions potentially associated with hypersensitivity which included, but were not limited to, pruritus, rash, urticaria, wheezing, or hypotension were reported in 1.5% (26/1775) of these subjects.
Hypertension
In clinical studies, hypertension was reported in 4% (67/1775) of subjects in clinical trials 1 and 2. Transient elevations in systolic blood pressure, sometimes occurring with facial flushing, dizziness, or nausea were observed in 6% (106/1775) of subjects in these two clinical trials. These elevations generally occurred immediately after dosing and resolved within 30 minutes. Monitor patients for signs and symptoms of hypertension following each Injectafer administration.
Laboratory Test Alterations
In the 24 hours following administration of Injectafer, laboratory assays may overestimate serum iron and transferrin bound iron by also measuring the iron in Injectafer.
ADVERSE REACTIONS
Adults
In two randomized clinical studies [Studies 1 and 2], a total of 1775 patients were exposed to Injectafer, 15 mg/kg of body weight, up to a maximum single dose of 750 mg of iron on two occasions, separated by at least 7 days, up to a cumulative dose of 1500 mg of iron. Adverse reactions reported by >2% of Injectafer-treated patients were nausea (7.2%); hypertension (4%); flushing (4%); injection site reactions (3%); erythema (3%); hypophosphatemia (2.1%); and dizziness (2.1%).
Pediatric
The safety of Injectafer in pediatric patients was evaluated in Study 3. Study 3 was a randomized, active-controlled study in which 40 patients (1 to 12 years of age: 10 patients, 12 to 17 years of age: 30 patients) received Injectafer 15 mg/kg to a maximum single dose of 750 mg (whichever was smaller) on Days 0 and 7 for a maximum total dose of 1500 mg; 38 patients evaluable for safety in the control arm received an age-dependent formulation of oral ferrous sulfate for 28 days. The median age of patients who received Injectafer was 14.5 years (range, 1-17); 83% were female; 88% White and 13% Black. The most common adverse reactions (≥4%) were hypophosphatemia (13%), injection site reactions (8%), rash (8%), headache (5%), and vomiting (5%).
Patients with Iron Deficiency and Heart Failure
The safety of Injectafer was evaluated in adult patients with iron deficiency and heart failure in randomized controlled trials FAIR-HF (NCT00520780), CONFIRM-HF (NCT01453608) and AFFIRM-AHF (NCT02937454) in which 1016 patients received Injectafer versus 857 received placebo. The overall safety profile of Injectafer was consistent across the studied indications.
Post-Marketing Experience
The following adverse reactions have been identified during post approval use of Injectafer. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following adverse reactions have been reported from the post-marketing spontaneous reports with Injectafer: cardiac disorders: tachycardia; general disorders and administration site conditions: chest discomfort, chills, pyrexia; metabolism and nutrition disorders: hypophosphatemia; musculoskeletal and connective tissue disorders: arthralgia, back pain, hypophosphatemic osteomalacia; nervous system disorders: syncope; respiratory, thoracic and mediastinal disorders: dyspnea; skin and subcutaneous tissue disorders: angioedema, erythema, pruritus, urticaria; pregnancy: fetal bradycardia.
USE IN SPECIFIC POPULATIONS
Pregnancy – Fetal/Neonatal Adverse Reactions
Severe adverse reactions including circulatory failure (severe hypotension, shock including in the context of anaphylactic reaction) may occur in pregnant women with parenteral iron products (such as Injectafer) which may cause fetal bradycardia, especially during the second and third trimester.
INDICATIONS
Injectafer® (ferric carboxymaltose injection) is indicated for the treatment of iron deficiency anemia (IDA) in adult and pediatric patients 1 year of age and older who have either intolerance or an unsatisfactory response to oral iron, and in adult patients who have non-dialysis dependent chronic kidney disease. Injectafer is also indicated for iron deficiency in adult patients with heart failure and New York Heart Association class II/III to improve exercise capacity.
You are encouraged to report Adverse Drug Events to American Regent, Inc. at 1-800-734-9236 or to the FDA by visiting www.fda.gov/medwatch or calling 1-800-FDA-1088.




